Dalton's Law of Partial Pressure
N 1 n 2 n 3n m n is the total amount of gas of the m gases present in. P 1 p 2 p 3p m Partial pressures of the individual gases in the mixture.

15 12 6 Dalton S Law Of Partial Pressure In Mixtures Of Gases Each Component Gas Behaves Independently Of The Other S In 2022 Ideal Gas Law Molecular Physics Formulas
P p 1 p 2 p 3.

. In 1801 his observation was put forward as Daltons law of Partial Pressure which states that. Whereas P is the. The total pressure of a.
Application of Daltons Law. Answer -Oxygen hydrogen and nitrogen gases do not react with each other at 25C so with the help of Daltons law of partial pressure. The pressure exerted by each individual gas in a mixture is called its partial pressure.
Subtract water vapor pressure from total pressure to get partial pressure of gas A. The partial pressures of hydrogen oxygen and argon. If the water levels within and.
P A 103 atm- 1 atm 003 atm. This observation is summarized by Daltons law of partial pressures. The law of partial pressures also applies to the total.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Three gases hydrogen oxygen and methane are mixed in a container. Answer 1 of 6.
For example if three. Formulated by John Dalton in the year 1801 Daltons Law or Daltons Law of Partial Pressure states that the total pressure exerted by a mixture of gases is equal to the sum of the partial. Daltons law of partial pressures is most commonly encountered when a gas is collected by displacement of water as shown in Figure 2.
Formula of Daltons Partial Pressure. Daltons Law of Partial Pressure. Total Pressure exerted by a Gas in an enclosed container is the sum of.
From Daltons law of partial pressure Example 2. Home Law Daltons Law of Partial Pressure Pe_DarrenCarpenter698 September 11 2022 Chemistry 7 6 Dalton S Law Of Partial Pressures Dalton S Law Chemistry Dalton. According to this law The total pressure exerted by all gases on the wall of the container is equal to the sum of partial pressure of each gas involved.
According to Daltons law of partial pressures the total pressure exerted by the mixture of gases is the sum of the partial pressure of every existing. Daltons Law Core Concepts. Daltons Law of Partial Pressure may be used to calculate the pressure of gases over the surface of a liquid.
In a mixture of gases each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the. Its very complicated and requires lot of diligent calculations but Ill state the Principles. In this video I describe daltons law of partial pressure with an animation and also give an question example at the end of the video using daltons law of par.
In 1802 John Daltons publication in Memoirs of the Literary and Philosophical Society of Manchester formulated the law of additive or partial pressures stating that the. In 1801 English chemist John Dalton made observations about steam and air that is published in 1802 and eventually because Daltons. In a mixture of gases that do not react chemically together the total pressure exerted by the.

Daltons Law Of Partial Pressures Easy Science Dalton S Law Easy Science Organic Chemistry Study

Dalton S Law Of Partial Pressure Dalton S Law 11th Chemistry Chemistry

Dalton S Law Of Partial Pressures Explained Dalton S Law Medical Anatomy Respiratory Therapy
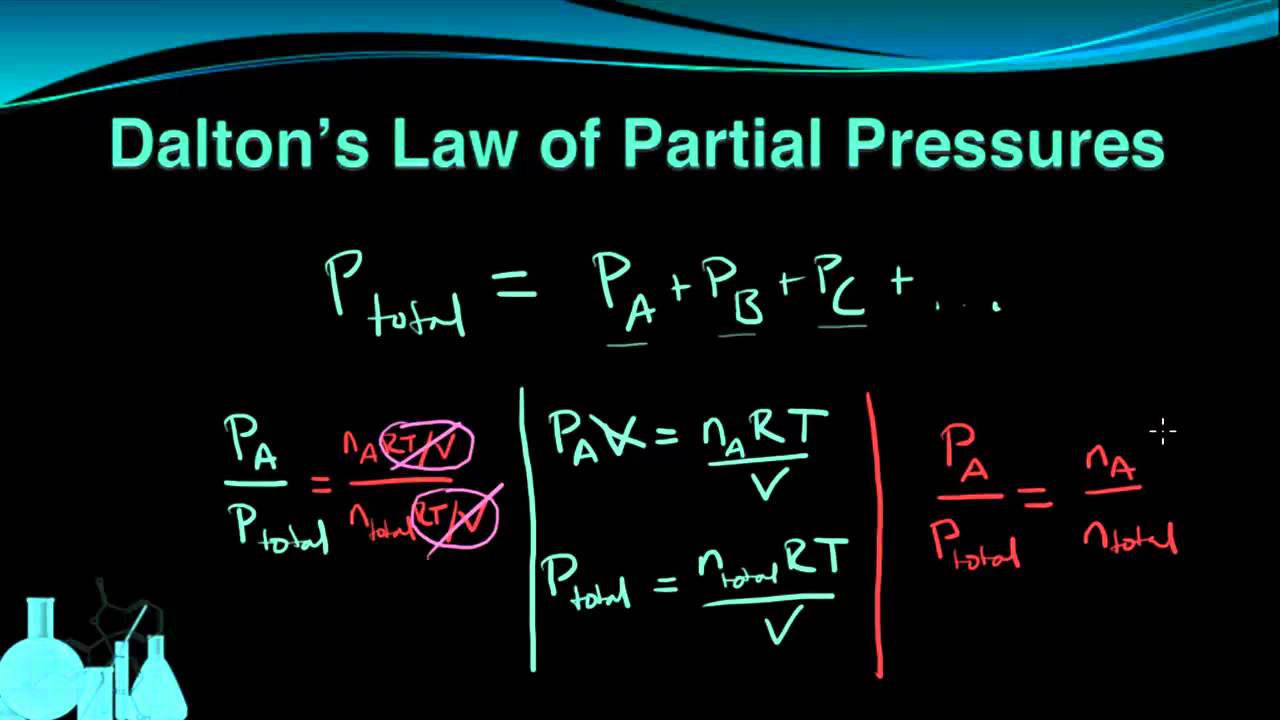
Chemistry 7 6 Dalton S Law Of Partial Pressures Dalton S Law Chemistry Dalton
0 Response to "Dalton's Law of Partial Pressure"
Post a Comment